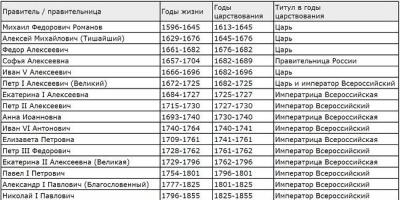
Distilled water- purified water, practically free of impurities and foreign inclusions. It is obtained by distillation in special apparatus - distillers.
Characteristics
Distilled water is standardized based on GOST 6709-72 “Distilled water”.
This parameter is also important for older lubricants, especially when working with large quantities where there is no known waste oil or burning smell or soot particles. Therefore, some oil analysis laboratories now perform conductivity measurements at different temperatures. It was originally designed to inspect aircraft kerosene to avoid accidents caused by jet fuel loading.
However, if the value is lower, there is a real possibility that this phenomenon may occur. Voltage within the system cannot be discharged through the ground wire. Fortunately, there are several other approaches to prevention. Install special antistatic filters instead of regular filter cartridges. Select or change the combination of system materials to prevent microporous formation despite electrostatic charge. Optimize flow diameter, reservoir retention time, or reservoir volumes to minimize charging potential.
- These filters can discharge or even prevent a charge from occurring.
- Use an oil with a different composition and higher conductivity.
Physical
The specific electrical conductivity of distilled water is usually less than 5 µS/cm. The conductivity of deionized water can be less than 0.05 µS/cm.
The most important parameter of drinking water for health
You can use a meter and probe or litmus paper to measure the pH of a sample. The more accurate but expensive of these methods is the meter and probe. pH meters are calibrated using special solutions or buffers with a known pH value. Calibration protocols can be found in the manufacturer's instructions, but a simplified protocol can be accessed through the pH assessment website environment at Washington State University.
Using litmus or pH paper is easier and cheap way pH measurements. This method uses special strips of paper that change color based on the pH of the sample solution. The strips have a variety of resolutions, from simple acid/base comparisons to fine resolution pH values.
Distilled water has pH =5.4-6.6
Peculiarities
Being very clean, in the absence of foreign mechanical inclusions, it can be overheated above the boiling point, or supercooled below the freezing point without undergoing a phase transition. The phase transition occurs intensively with the introduction of mechanical impurities or shaking.
Electrical conductivity can also be measured using a meter and probe. The probe consists of two metal electrodes located at a distance of 1 cm from each other. It is supplied at the electrodes constant pressure, which results in an electrical current flowing through the aqueous sample. Since the current flowing through water is proportional to the concentration of dissolved ions in the water, electrical conductivity can be measured.
Introduction and Definitions
Manufacturer's instructions and guidelines should be followed. These pH strips can measure pH over a series of ranges by placing a sample on the strip and comparing its color change to the colors on the box that match a certain value pH. The bar on the left measures pH 0-7 and shows the results of a strong acidic sample; the central band represents the pH range 5-10 and shows the results of a sample of buffer solution 97; the bar on the right measures a wide range and shows the results of a 10% water bleach solution.
Usage
Distilled water is used to adjust the electrolyte density, safe battery operation, flushing the cooling system, diluting coolant concentrates, and for other household needs. For example, to correct the freezing point of antifreeze windshield washer fluid and color photo printing.
Place a drop of the sample on the paper - make sure you drop or pour the sample onto the paper and not immerse the paper in the sample as the latter can contaminate the sample. When using litmus paper, the paper will be colored red or pink color, if the sample is acidic, and the blue paper indicates the base sample. Calibration procedures vary among instruments, so it is recommended that you follow the manufacturer's recommendations. Calibration procedures vary depending on the instrument, so following the manufacturer's instructions is highly recommended. Observe the color change on the paper. . The latter method can be done using a kit containing the necessary chemicals.
Harm to human health
Constant consumption of distilled water causes irreparable harm to human health due to the creation of an imbalance of water-salt balance. Imbalance occurs when the pH - the pH value of human blood and distilled water - does not match.
The most important parameter of drinking water for health
pH - pH value
pH is a hydrogen index, (from the Latin words potentia hydrogeni - the strength of hydrogen) - a measure of activity (in the case of dilute solutions, reflects the concentration) of hydrogen ions in a solution, quantitatively expressing its acidity, calculated as a negative (reversed) decimal logarithm of the concentration of hydrogen ions, expressed in moles per liter: pH = -log. Those. pH is determined by the quantitative ratio of H+ and OH- ions in water, formed during the dissociation of water. (A mole is a unit of measurement for the amount of a substance.) In distilled water, the pH is<7. Растворение в воде различных солей приводит к изменению значения рН.
When the concentrations of both types of ions in a solution are the same, the solution is said to be neutral. When an acid is added to water, the concentration of hydrogen ions increases, and the concentration of hydroxide ions correspondingly decreases; when a base is added, on the contrary, the content of hydroxide ions increases, and the concentration of hydrogen ions decreases. When > the solution is said to be acidic, and when > it is alkaline.
The body balances the pH of internal fluids, maintaining values at a certain level. The acid-base balance of the body is a certain ratio of acids and alkalis in it, which contributes to its normal functioning. The acid-base balance depends on maintaining relatively constant proportions between intercellular and intracellular waters in the tissues of the body. If the acid-base balance of fluids in the body is not constantly maintained, normal functioning and preservation of life will be impossible.
Optimal pH of drinking water = 7.0 to 8.0.
According to Japanese researchers, drinking water with a pH above 7 increases the life expectancy of the population by 20-30%.
The electrode method uses a special series of probes, two of which send an electrical current through the ground, and two of which measure the voltage drop. In general, pH readings between 1-6 are considered acidic, 7 neutral, and 8-14 basic. Relatively dilute waters, such as distilled water or glacial meltwater, have low electrical conductivities ranging from zero to the microseima range, while moderate streams and lakes, especially those that have a significant contribution to groundwater, usually have higher electrical conductivity.
MINISTRY OF EDUCATION AND SCIENCE
RUSSIAN FEDERATION
FEDERAL AGENCY FOR EDUCATION
State educational institution
"KAZAN STATE
ENERGY UNIVERSITY"
DETERMINING THE SPECIFIC CONDUCTIVITY OF WATER USING A PWT CONDUCTOMETER Hanna Instruments
More about electrical conductivity
Radiation exposure radiation exposure. Absorbed Dose Metric Prefixes Data Communication Printing and Digital Imaging Devices Wood Volume Measurement Molar Mass Calculator Periodic Table. Electrical conductivity or specific conductivity is a measure of a substance's ability to conduct or transport electrical current. electric charge. This is the ratio of current density to voltage electric field. If we consider a 1 meter cube of conductive material, then the conductivity will be equal to the electrical conductivity measured between the opposite faces of that cube.
Laboratory work according to the course
(4 hours)
"Environmental audit in the energy sector
and industry"
Kazan
2010
Determination of the electrical conductivity of water using a PWT conductometer Hanna Instruments
Goal of the work
1. Get acquainted with the design and operating principle of the PWT Hanna Instruments conductivity meter.
State educational institution
Conductivity and conductivity are related by the formula. This formula can be used with any cylindrical or prismatic conductor. Note that this formula can also be used for a cuboid because it is a rectangular prism. Electrical conductivity is the reciprocal of electrical resistance. IN English language the words conductivity and conductivity are so similar that they are often used interchangeably. Meanwhile, of course, they have a different meaning. Conductivity is an extrinsic property of a given conductor or device, and conductivity is an inherent property of the material from which that conductor or device is made.
2. Learn to determine the electrical conductivity of water by conductometry using a PWT Hanna Instruments conductometer.
3. Get acquainted with the structure and operating principle of a distiller and double-distiller, study the change in the electrical conductivity of water before and after distillation.
Work assignment
1. Get to know the operating principle of the PWT Hanna Instruments conductivity meter;
For example, the conductivity of copper is always the same, no matter how an object made of copper changes in shape or size, and the conductivity copper wire depends on its length, diameter, mass, shape and several other factors. Of course, similar objects made from higher conductivity materials will have greater conductivity.
Note that in English the same unit form "siemens" is used for both singular and plural forms. This division is named after the German inventor, industrialist and scientist Werner von Siemens. Electrical conductivity varies from highly resistive materials such as glass or acrylic glass, to semiconductors that have different conductivity in different conditions for extremely conductive materials such as silver, copper or gold.
2. Get acquainted with the structure and operating principle of the distiller;
3. Measure the electrical conductivity of water before and after distillation;
4. Describe the progress of the work;
5. Draw up a protocol of measurement results;
6. Answer the security questions.
Equipment and reagents
1. conductivity meter PWT Hanna Instruments;
2. distiller;
Electrical conductivity is determined by the number of charge carriers such as electrons or ions, the speed at which they move, and the amount of charge they carry. The conductivity of aqueous solutions, such as plating baths, lies between these extremes. Another example of electrolytes with moderate conductivity are our bodily fluids: blood, plasma, lymph, etc.
The conductivity of metals, semiconductors and dielectrics is discussed in detail in the article "More about Electrical Resistance, Electrical Resistance and Electrical Conductivity and Electrical Conductivity". In this article, we will discuss in more detail the electrical conductivity of electrolytes and their measurement methods and equipment. We describe several experiments using a low-cost conductivity measurement device.
3. redistiller;
4. beakers with a capacity of 150-200 ml.
Theoretical part
General information
Electrical conductivity is the ability of an aqueous solution to conduct electric current, expressed in numerical form. Electrical conductivity natural water depends on the degree of mineralization (concentration of dissolved mineral salts) and temperature. Therefore, by the electrical conductivity of water, one can judge the degree of mineralization of water. Natural water is a solution of mixtures of strong and weak electrolytes. The mineral part of water consists of sodium ions (Na+), potassium (K+), calcium (Ca2+), chlorine (Cl-), sulfate (SO42-), bicarbonate (HCO3-). It is these ions that determine the electrical conductivity of natural waters. Electrical conductivity depends on: ion concentration, nature of ions, solution temperature, solution viscosity.
Electrolytic conductivity and its measurement
The conductivity of aqueous solutions in which electric current is carried by charged ions is determined by the number of charge carriers, the speed of their movement and the charge they carry. Therefore, in most aqueous solutions, a higher concentration will result in more ions and therefore higher conductivity. However, after reaching the maximum concentration, conductivity may begin to decrease with increasing concentration. Therefore, two different concentrations of the same salt can have the same conductivity.
Pure water as a result of its own dissociation, it has a specific electrical conductivity at 25 C equal to 5.483 μS/m.
Methods for measuring the electrical conductivity of water
To determine the electrical conductivity of water, the conductometric method is usually used.
Conductometry- (from English conductivity - electrical conductivity and Greek metreo - measure), electrochemical method of analyzing solutions chemical substances and natural waters, based on measuring their electrical conductivity. The principle of conductometric analysis is the change chemical composition environment or concentration of a certain substance in the interelectronic space. The advantages of conductometry include: high sensitivity, sufficiently high accuracy, simplicity of methods, availability of equipment, the possibility of studying colored and turbid solutions, as well as automating the analysis. To measure the electrical conductivity of aqueous solutions, melts, colloidal systems, a special device is used - conductivity meter.
Big Polytechnic Encyclopedia
Temperature also affects conductivity because at higher high temperatures the ions move faster, increasing conductivity. Pure water does not conduct electricity well. Conductivity various solutions is given in the table below. To determine the conductivity of a solution, a conductivity or resistance meter is typically used and the measured value is then manually or automatically converted to conductivity. This includes the area of the electrodes and the separation distance between the two electrodes.
Applications of conductometry
Conductometers are used to control the electrical conductivity of liquid media in technological processes chemical, petrochemical production, energy facilities (CHP, nuclear power plant), where electrical properties liquids characterize product quality.
The sensors are very simple: they contain a pair of electrodes immersed in an electrolyte solution. This formula works well when the area of the electrodes is much larger than the separation between them, since in this case most of electric current flows directly between the electrodes. Note that cells with small, widely spaced electrodes have cell constants of 0 cm-1 or more, while cells with large, closely spaced electrodes have cell constants of 1 cm-1 or less. Cell constant various devices for conductivity measurements varies from 01 to 100 cm-1.
Assessing the quality of distilled water by specific electrical conductivity is a textbook operation. Distilled water must have an electrical conductivity of no more than 10-6 Sim (ohm-1).
Description of the conductivity meter PWT Hanna Instruments
The PWT Hanna Instruments conductometer is a device designed for express determination of the electrical conductivity of water. Can be used both in laboratories and in field conditions. Main features of the device: manual one-point calibration, automatic temperature compensation. Electrical conductivity measurements are carried out using an OK-102 conductivity meter, which allows you to immediately determine the values of specific electrical conductivity in Siemens.
The following formula is used to obtain conductivity from measured conductivity. The cell constant is usually not calculated, but rather measured for a specific measuring device or installation using a solution of known conductivity. This measured value is entered into the meter, which automatically calculates the conductivity from the measured conductivity or resistance.
The simplest way to measure conductivity is to apply a voltage across two flat electrodes immersed in a solution and measure the resulting current. This is called the potentiometric method. However, everything is not as simple as it seems. This will lead to an increase in polarization resistance on the electrode surfaces, which in turn can lead to erroneous results. If we try to measure the resistance of, for example, a sodium chloride solution using a multimeter, we can clearly see that the reading on the display increases quite quickly.
DIV_ADBLOCK169">
Preparation of distilled water
Distillation is the separation of multicomponent liquid mixtures into fractions that differ in composition by partial evaporation of the mixture and condensation of the resulting vapors. The condensate thus obtained is enriched with low-boiling components, the remainder liquid mixture- high boiling. Distillation produces a purer, more refined and concentrated product. Distilled water is purified water that has removed mineral salts, organic substances, ammonia, carbon dioxide and other impurities dissolved in it. It is obtained by distillation in special apparatus - distillers.
Laboratory work" href="/text/category/laboratornie_raboti/" rel="bookmark">laboratory work To obtain distilled water, a DE-4 distiller and a PURATOR-MONO redistiller are used.
Progress
Pour tap water into a 150-200 ml beaker. Turn on the conductivity meter and place it in the volume under study; record the measurement results in the protocol.
Pour the water obtained using the DE-4 distiller into a beaker with a capacity of 150-200 ml. Turn on the conductivity meter and place it in the volume under study; record the measurement results in the protocol. Repeat the operation with water obtained using a double distiller.
Measurement protocol
Control questions
1. What does the electrical conductivity of water depend on?
2. What methods for determining the specific electrical conductivity of water do you know?
3. What device is used to determine the specific electrical conductivity of water?
5. Name the area of application of conductometry.
6. How is distilled water obtained?