Carbon chain names
Chain Main Side (hydrocarbon radical) C met methyl C2 ethyl C3 propyl C4 but butyl C5 pent pentyl C6 hex hexyl C7 hept heptyl C8 oct octyl C9 non nonyl C10 dec decyl
Designation of the degree of saturation of bonds
С-С С=С С≡С (here is a triple bond between atoms)
Names of characteristic groups of organic compounds
Compound class Characteristic Prefix Suffix group Carboxylic acids -COOH carboxycarboxylic acid Carboxylic acids -(C)OOH - oic acid Sulfonic acids -SO3H sulfosulfonic acid Aldehydes -CHO formyl carbaldehyde Aldehydes -(C)HO oxo al Ketones >(C)=O oxo he Alcohols -OH hydroxy ol Phenols -OH hydroxy ol Thiols -SH mercapto thiol Amines -NH2 amino amine Ethers -OR alkoxy, aroxy - Halogen derivatives -F, -Cl, -Br, -I fluorine, chlorine, bromine, iodine - Nitro compounds -NO2 nitro -
The carbon atom enclosed in parentheses is part of the name of the main carbon chain.
The arrow shows an increase in the seniority of characteristic groups.
Names of aromatic compounds
CH 3 -OH -COOH
C 6 H 6 C 6 H 5 CH 3 C 6 H 5 OH C 6 H 5 COOH
benzene toluene phenol benzoic acid
Names of some hydrocarbon radicals
(CH 3) 2 CH-CH 3 CH 2 (CH 3)CH- (CH 3) 2 CH-CH 2 - (CH 3) 3 C-
isopropyl tues-butyl isobutyl rubs-butyl
CH 2 =CH- CH 2 =CH-CH 2 - HC≡C- (with a triple bond)
vinyl allyl ethynyl
phenyl benzyl
Numeric prefixes
(indicate the number of identical structural elements)
1 mono monoxide
3 three tris
4 tetra tetrakis
5 penta pentakis
6 hexa hexakis
At composing the name substances according to its structural formula(and vice versa), you must perform the following steps sequentially:
1. Find the main one (by seniority) characteristic group and choose a designation for it in the suffix.
2. Find main carbon chain (cycle), including the main characteristic group, and number it from the end of the chain closer to which the senior group is located. If there are several such possibilities, then you need to take into account the presence of:
a) other characteristic groups (by seniority);
b) double bond;
c) triple bond;
d) other substituents (in alphabetical order).
3. To the name of the main circuit add suffix, denoting degree of saturation of connections. If there are several multiple bonds in the molecule, their number must be indicated in the suffix, and after the suffix - in Arabic numerals their position in the carbon chain. Next, the suffix includes the name of the senior characteristic group, indicating its position in Arabic numerals.
4. With consoles (prefixes) designate deputies(side chains, minor characteristic groups) and arrange them alphabetically. The position of the substituent must be indicated with a number before the prefix.
5. Arrange digital set-top boxes, indicating the number of repeating structural elements (they are not taken into account when alphabetizing prefixes).
6. Arrange punctuation marks: all numbers are separated from words by a hyphen, and from each other by commas.
The generally accepted nomenclature for organic substances is the IUPAC systematic nomenclature.
The name is based on the name of the main carbon chain of the molecule, which may not be the longest, but must necessarily include multiple bonds (or their maximum number) and the highest functional group (or their maximum number) of the compound. To designate functional groups in names, appropriate prefixes (prefixes) or suffixes are used (see Appendix A, tables A.1 and A.2). The formulas and names of the main functional groups must be memorized.
In the name of an organic substance, as in any word, one can distinguish a prefix, a root, a suffix and an ending. Each part of the word carries its own semantic load (Table 2.1).
Table 2.1 – Naming an organic substance
| Name of organic substance | ||||
| CONSOLE | ROOT | SUFFIX | ENDING | |
| Substituents (radicals, junior functional groups, senior functional groups that are not included in the main chain) | Number of carbon atoms of the main chain | The degree of saturation of C-C bonds in the main chain | Senior Functional Team | |
| ü Alphabetical order is maintained. ü Using Arabic numerals, the place of each group in the main chain is indicated. ü For identical substituents, multiplying prefixes are used di-, tri-, tetra-, penta- etc. | Meth-Et-Prop-But-Pent-Hex-Hept-Oct-Non-Dec-(dec-) | 1 C 2 C 3 C 4 C 5 C 6 C 7 C 8 C 9 C 10 C | ü -an (single bonds only); ü-ene (1 double bond); ü-diene (2 double bonds) ü-yne (1 triple bond), etc. ü For multiple bonds, the location in the chain is indicated. | ü Arabic numerals indicate the location of the functional group in the chain (except for aldehyde and carboxyl groups). ü For identical groups, multiplying prefixes are used di-, tri-, tetra-, penta- etc. |
· Root The word corresponds to the name of the number of carbon atoms of the main carbon chain.
· Suffix indicates the degree of saturation of the main chain. Yes, suffix -an indicates the absence of multiple bonds in the main carbon chain. A double bond is indicated by the suffix -en, triple – suffix -in. To denote several similar multiple connections, Greek numerals are used di-, tri-, tetra-, penta-, etc. For multiple bonds, their place in the main chain must be indicated, using Arabic numerals located after the suffix.
· Ending corresponds to the name of the senior functional group indicating its place in the main carbon chain. Greek numerals are used to denote several functional groups of the same type di-, tri-, tetra-, etc.
· IN prefix (prefix) hydrocarbon radicals and functional groups not indicated at the end of the name are listed in alphabetical order, indicating their place in the main carbon chain. Multiplying prefixes are used for identical radicals or functional groups di-, tri-, tetra-, etc.
Algorithm for compiling a systematic name
organic matter
1. Determine the senior functional group and class of the compound. If a substance does not contain functional groups and consists only of carbon and hydrogen atoms, then it is a hydrocarbon. Functional groups in descending order of precedence are given in Appendix A (Table A.1).
2. Based on the presence or absence of multiple bonds between carbon atoms, we determine the degree of saturation of the carbon chain.
3. Select the main carbon chain.
· The main carbon chain begins and ends with a primary carbon atom, i.e. a carbon atom that forms bonds with only one carbon “neighbor”.
· For saturated organic compounds, it should be the longest possible, and should also include the highest functional group (or the maximum number of them).
· In saturated hydrocarbons, choose the longest carbon chain. If there are several such chains, then we select the most branched chain containing radicals with a smaller volume.
· For unsaturated compounds, the main chain must necessarily include a multiple bond or their maximum number, as well as a senior functional group (or their maximum number).
· In unsaturated hydrocarbons, the main chain may not be the longest, but it must include a multiple bond (or their maximum number).
· In aromatic hydrocarbons with a branched side chain, we select the main chain in the side branch, considering the aromatic ring as a radical.
· Carbocyclic and heterocyclic structures are usually included in the main chain.
4. Number the selected main carbon chain.
· We start numbering carbon atoms from the end to which the highest functional group (or their maximum number) is closest.
· If the functional group is equidistant from the ends of the chain, then the direction of numbering is determined by the position of the multiple carbon-carbon bond.
· In saturated hydrocarbons, we start numbering the chain from the end to which the structural radicals (or their maximum number) are located closer, taking into account their seniority. The fewer carbon atoms a radical contains and the less branching it has, the older the radical.
· In unsaturated hydrocarbons, the direction of numbering is determined by the multiple bond, with the double bond being older than the triple bond.
· In arenas, the benzene ring is numbered so that the substituents receive the lowest possible numbers. The beginning of numbering is determined by the highest radical.
· In a heterocycle, the beginning of numbering is determined by the heteroatom.
5. Make up the name of the organic substance.
Remember:
· After suffixes indicating the presence of multiple carbon-carbon bonds in the main chain, a hyphen is placed, followed by Arabic numerals indicating their position and separated by commas.
· The position of senior functional groups is indicated by Arabic numerals, which are placed after the corresponding ending and separated from it by a hyphen.
· After graduations -al or -oic acid numbers are not needed, because The numbering of the main chain begins with the carbon atoms of the corresponding functional groups.
· The number of substituents indicated in the name must coincide with the number of digits indicating their position in the chain.
Multiplying prefixes are used for identical substituents di-, tri-, tetra-, penta-, etc.
Examples of naming
1) S – S – S – S – S – S – S – S
S – S – S S – S S S
Note. This example shows only the carbon skeleton of the molecule. Before writing the name of the compound, it is necessary to add H atoms to the given formula, taking into account the fact that the C atom in organic compounds is 4-valent.
CH 2 –CH 2 –CH–CH 2 –CH 2 –CH 2 –C–CH 3
CH 3 –CH –CH 3 CH 2 –CH 3 H 3 C CH 3
1. The substance whose structural formula is given above is a hydrocarbon.
2. Between carbon atoms all bonds are single. Therefore, it is a saturated hydrocarbon - an alkane.
3. Select the longest and most branched chain, starting and ending with its primary atom C.
4. We number the selected chain from the end to which the methyl radicals are located closer (the methyl radical is older than the ethyl radical).
CH 2 –CH 2 –CH–CH 2 –CH 2 –CH 2 –C–CH 3
10 CH 3 – 9 CH– CH 3 CH 2 –CH 3 H 3 C CH 3
Methyl ethyl methyl methyl
radicals
5. Make up the name of the hydrocarbon:
2) CH 2 =C–CH 2 –CH–CH 2 –CH 3
C 2 H 5 CH 3
1. This compound is a hydrocarbon.
2. There is 1 double bond in the molecule. Therefore it is an alkene.
3. We select the main chain so that it contains a double bond and the chain is as long as possible.
4. The numbering of the chain starts from the side to which the multiple bond is located closest.
CH 2 =C–CH 2 –CH–CH 2 –CH 3
Ethyl C 2 H 5 CH 3 methyl
Nomenclature is a set of names of individual chemical substances, their groups and classes, as well as rules for compiling their names. The name of a substance must reflect not only its qualitative and quantitative composition, but also clearly show its chemical structure; the name must correspond to a single formula of structure. The scientific development of the nomenclature of organic compounds began in the mid-19th century, its further development led to the modern nomenclature developed by an international commission according to the nomenclature of organic compounds of the International Union of Pure and Applied Chemistry (IUPAC). The structure of molecules of organic compounds is expressed using complex words-names, including the following fragments:
a) designation of carbon chains C n: C – “met”, C 2 – “eth”, C 3 – “prop”, C 4 – “but”, C 5 and subsequent ones – roots of Greek numerals – “pent”, “hex” ", "hept", "oct", "non", etc.
b) the designations of side chains - hydrocarbon radicals - consist of the above names of carbon chains and the ending “il” (methyl, ethyl, propyl, etc.).
c) designation of the nature of the bond between atoms - “an” - single, “en” - double, “in” - triple bond
d) designation of characteristic groups, and the name of the same group differs depending on the way the name is constructed and on the seniority of the group - the older one is mentioned in the suffix, the younger one in the prefix.
e) multiplying prefixes - “di”, “tri”, “tetra” and their modified forms - “bis”, “tris”, etc., showing the number of identical structural elements
f) locants - numbers or letters showing the order of articulation of the constituent parts of the molecule
g) separators – hyphens, commas, periods, parentheses.
When compiling a name according to the IUPAC nomenclature (as well as when constructing a structural formula for a name), the following rules are consistently followed:
1) Find the senior characteristic group and choose a designation for it in the suffix (Table 1).
2) The main chain or main cyclic structure to which the main characteristic group is adjacent is identified and named. In this case, they are guided by the following seniority of structural fragments: characteristic groups in order of decreasing precedence (those for which mention is provided in the suffix), double bond, triple bond, other prefix substituents in alphabetical order.
3) Determine the multiplicity of bonds, using the suffixes “an”, “en” and “in” to indicate it, and in the carbo- or heterocyclic series - the prefixes “dihydro”, “tetrahydro”, etc.
4) Determine the nature of the substituents (side chains and junior substituents) and arrange their designations in alphabetical order in the prefix part of the name.
5) Define multiplying prefixes, taking into account that they do not affect the alphabetical order of prefixes.
6) The main chain or main cyclic structure is numbered, with the main characteristic group having the lowest number. Locants are placed before the name of the main structure (2-butanol, 3-hexanol), before prefixes (3-chlorobutanol-1), before or after the suffix to which they relate (3-hexen-2-one, 3-hexanone-2) .
7) Make up a name from the above components, using the necessary separators.
Table 1.
CLASSES OF ORGANIC COMPOUNDS AND NAMESCHARACTERISTIC GROUPS
(in descending order of precedence)
| CLASS | FUNCTIONAL GROUP | NAME | |
| in the prefix | in the suffix | ||
| Cations | -X+ | they are about | this one |
| Carboxylic acids | -COUN -(C)UN * | carboxy - | carboxylic acid oic acid |
| Sulfonic acids | -SO3H | sulfo | sulfonic acid |
| Amides | -CONH 2 -(C)ONH 2 | carbamoyl - | carboxamide amide |
| Nitriles | -CN -(C)N | cyano - | carbonitrile nitrile |
| Aldehydes | -SNO -(C)BUT | formed oxo | carbaldehyde al |
| Ketones | C=O | oxo | He |
| Alcohols, phenols | -HE | hydroxy | ol |
| Thiols | -SH | mercapto | thiol |
| Amines | -NH 2 | amino | amine |
| Double bond | = | - | en |
| Triple bond | - | in | |
| Ethers** | -OR | alkoxy, aroxy | - |
| Halogen derivatives | -F -Cl -Br -I | fluorine chlorine bromine iodine |
- - - - |
| Nitroso compounds | -NO | nitroso | - |
| Nitro compounds | -NO 2 | nitro | - |
| Diazo compounds | -N 2 | diazo | - |
| Azids | -N 3 | azido | - |
**Characteristic groups of ethers and subsequent classes are listed in prefixes alphabetically and not by precedence
Example 1.
We determine the longest chain of carbon atoms (it will be the main one) and number the carbon atoms. This compound has a characteristic amino group and a side chain containing one carbon atom. The prefix part of the name, given that the amino group is older than the side chain: 2-methyl-3-amino-... The main chain contains 5 carbon atoms, so the main part of the name is "pent" (five): 2-methyl-3-aminopent... Presence of a double bond indicated by the suffix “ene”: 2-methyl-3-aminopentene-... The double bond begins at the carbon atom of the main chain with serial number 1, so the final word is: 2-methyl-3-aminopentene-1.
The name of the compound is based on the root word denoting a saturated hydrocarbon with the same number of atoms as the main chain (for example, met-, et-. pro p-, but-, pent-, hex-, etc.). This is followed by a suffix characterizing the degree of saturation, -an if the molecule has no multiple bonds, -en if there are double bonds and -ni for triple bonds, for example, pentane, pentene. If there are several multiple bonds in the molecule, then the number of such bonds is indicated in the suffix, for example: -diene, -triene, and after the suffix the position of the multiple bond must be indicated in Arabic numerals (for example, butene-1, butene-2, butadiene-1,3) :
CH 3 -CH 2 -CH=CH 2 CH 3 -CH=CH-CH 3 CH 2 =CH-CH=CH 2
butene-1 butene-2 butadiene-1,3
Next, the suffix contains the name of the oldest characteristic group in the molecule, indicating its position with a number. Other substituents are designated using prefixes. Moreover, they are listed not in order of seniority, but alphabetically. The position of the substituent is indicated by a number before the prefix, for example: 3-methyl; 2-chloro, etc. If a molecule has several identical substituents, then the number of them is indicated before the name of the corresponding group (for example, dimethyl-, trichloro-, etc.). All numbers in the names of molecules are separated from words by a hyphen, and from each other by commas. Hydrocarbon radicals have their own names.
Saturated hydrocarbon radicals:
methyl ethyl propyl isopropyl
Butyl sec-butyl
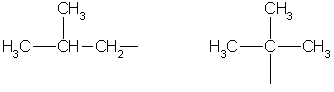
isobutyl tert-butyl
Unsaturated hydrocarbon radicals:
CH 2 = CH- HC - C- CH 2 = CH-CH 2 -
vinyl ethynyl allyl
Aromatic hydrocarbon radicals:

phenyl benzene
Let's take the following connection as an example:

The choice of chain is unambiguous, therefore, the root of the word is pent, followed by the suffix -en, indicating the presence of a multiple bond; the numbering order ensures that the oldest group (-OH) has the smallest number; the full name of the compound ends with a suffix indicating the senior group (in this case, the suffix –o l indicates the presence of a hydroxyl group); The position of the double bond and hydroxyl group is indicated by numbers.
Therefore, the given compound is called penten-4-ol-2.
The trivial nomenclature is a set of unsystematic historically established names of organic compounds (for example: acetone, acetic acid, formaldehyde, etc.). The most important trivial names are introduced in the text when considering the corresponding classes of compounds.
Rational nomenclature allows the name of a substance to be based on its structure, with a simpler compound chosen as a prototype. The following examples illustrate the method of such construction:
trimethylmethane acetylacetone phenylacetic acid
SUBJECT: Nomenclature of organic compounds .
Nomenclature is the language of organic chemistry, which is used to convey the structure of organic compounds in the names; it is a system of rules that allows you to give unambiguous names to each individual connection.
There are different systems of nomenclature for organic compounds: historical, rational, modern, or international.
The main one is the international systematic nomenclature, or Geneva. Its basic principles were adopted at the international congress
chemists in Geneva in 1892. Later changes were made to it, and the modern nomenclature bears the abbreviation IUPAC.
In order to name a substance, you need to select the longest chain, and then follow the following rules:
THEORETICAL PART.
The names of compounds consist of three parts :
1. the first part indicates the number of carbon atoms in the chain and its
name (see below frommeth - before Dec -)
If a carbon chain contains a certain number of carbon atoms, then the name of the chain will begin in accordance with this table; see the endings of the names accordingly in paragraph 2.
one carbon atom meth- six carbon atoms hex-
two carbon atom =this- seven carbon atoms hept-
three carbon atom =prop- eight carbon atoms Oct-
four carbon atom but- nine carbon atoms non-
five carbon atoms =pent- ten carbon atoms dec(dec-)
2. the ending (the particle at the end of the name) indicates the presence of single or multiple bonds.
a) particle -an- means that all connections in the carbon chainsingle,
b) - en one double bond ,
G) -diene – means that the carbon chain hastwo double bonds,
d) - in – means that the carbon chain hasone triple bond.
After indicating the multiplicity of the bond, you need to indicate its location in the chain by writing the number of the carbon atom at which it begins.
3. If a substance has a functional group, then particles are added to its name:
A) - ol – means hydroxo group- HE
b) -amine – means amino group– N.H. 2 ABOUT
//
V) -al – means carbonyl group (aldehydes– S – N ),
G) -He means carbonyl group (ketones– C = O ),
∣
d) -new acid means the presence of a carboxyl group in the chain–COUN
After designating a functional group, you need to indicate its location in the chain by writing the number of the carbon atom at which it begins.
4. If radicals are attached to the chain, then you need to name them by adding a particle– silt to the name of the number of carbon atoms that are included in a given radical, indicating the number of the carbon atom to which it is attached.
Radicals are always listed ahead of the carbon chain name.
5. When numbering a chain, you must remember that you need to start it with that
side to which it is closer (taking into account seniority):
a) functional group
b) multiple connection
c) the presence of a radical
PRACTICAL PART
Examples of substance names
1) 1 CH 3 - 2 dc - 3 CH 2 - 4 CH 3 y 2nd atom radical –2- methyl
| chain of 4 carbon atoms –but-
CH 3 all connections are single --an
no functional groups
2-met - silt but-an
2-methylbutane
2) 1 CH 3 - 2 dc - 3 dc - 4 CH 3 y 2nd and 3rd atom two radical –
| | 2,3- di met-yl
CH 3 CH 3 chain of 4 carbon atoms –but– all connections are single -–an
No functional groups
2,3-di – methyl but-ane
2,3-dimethylbutane
3) 1 CH 3 - 2 dc - 3 CH 2 - 4 CH - 5 CH 3 y 2nd atom radical –2-met-yl
| | at 4th atom radical –4-et-il
CH 3 C 2 H 5 chain of 5 carbon atoms –pent-
All connections are single -–an
no functional groups
2 met-il 4 et - il pent-an
2-methyl 4-ethylpentane
4) 1 CH 3 - 2 dc 3 dc - 4 CH 2 - 5 CH 3 y 4th atom radical –4th floor – silt
| chain of 5 carbon atoms –pent-
CH 2 one double bond on the 2nd atom – -en -2
| no functional groups
CH 3 4- et-yl pent - en-2
4-ethylpentene-2
5) 1 CH 3 - 2 dc - 3 CH 2 - 4 CH 3 no radicals
| chain of 4 carbon atoms –bottle -
OH all connections are single -–an
at the 2nd atom functional hydroxo group-–ol -2
but-an –ol–2
Butanol –2
C 3 H 7 O
| //
6) 5 CH 3 - 4 C 3 CH – 2 CH 2 - 1 C - HE
at 4 th atom radical– 4 -prop - silt
chain of 5 carbon atoms –pent-
one double bond at the 3rd atom – -en -2
there is a functional carboxyl group –-oic acid
4 – prop - silt pent – en - 3 - oic acid
4-propylpentene-3-oic acid
Drawing up formulas based on the names of substances
To create a chemical formula based on the name of a substance, the following procedure is necessary:
1. divide the given name of the substance into parts according to paragraphs 1 – 6 above
2. make a carbon chain according to the given number of atoms
3. enter the functional group
4. Considering the numbers, put the connections in it
5. Enter the radicals according to the indicated numbers
6. fill in the missing hydrogen atoms
For example:
Draw an IUPAC representation of the following substance: 2-methyl-3-ethylbuten-2-ol-1
1) 2-met-yl-3-eth-yl-but-en-2-ol-1
2) the largest number meansbut - means a chain of 6 carbon atoms
3) -ol-1 means hydroxo group at the first carbon atom
4) -en-2 means double bond at the second carbon atom
5) 2-methyl – 3-ethyl means two radical (first and second) second and third carbon atoms
Therefore, the formula looks like this: 5 CH 3 - 4 CH 2 - 3 C = 2 C - 1 CH 2 - HE
| |
C 2 H 5 CH 3
Training tasks:
1. Give the following IUPAC names:
1) CH 3 - C C - CH 2 - CH 3 2) CH 2 = CH - CH = CH 2
3) CH 3 - C = CH - CH - CH 3 4) CH 2 CH - WITH - CH 3
| | ‖
CH 3 CH 3 O
5) CH 3 - CH - CH - CH 2 - CH = CH - WITH - N
| |
Cl CH 3
2. Draw the following substances according to IUPAC:
a) 4-ethylpentine-2 b) 3-propylbutanone