Rust is dangerous disease for metal, it appears from the reaction of oxygen and carbon dioxide with water. When rust stains initially appear on the metal, it must be cleaned and treated with a protective agent. Can be cleaned chemical type using orthophosphoric acid.
Orthophosphoric acid is a powder that is diluted with water and is used in various industries, as well as medicine. The acid is used in diluted form, 85% aqueous solution, which does not have a pungent odor. Acid is used in production household products which are used to remove rust. It is also added to primer mixtures for metal.After surface treatment phosphoric acid, a protective layer is formed that prevents the material from destruction.
Basic precautions when working with phosphoric acid
When removing rust chemically, it is necessary to protect the respiratory tract and hands; for this, use a respirator and gloves. Since the evaporation of acid can cause burns to the respiratory tract and skin. In addition, the product is a fire hazard, so when working it is necessary to ensure good ventilation in the room.If acid gets on the skin, you must immediately rinse the area with plenty of water for about 15 minutes. If the damage is significant, you should immediately visit the hospital. Do not wipe the product with a napkin or towel.
How to properly remove rust using phosphoric acid
The advantage of phosphoric acid is that it removes loose rust and also forms a thin layer that serves as protection. Acid can eat away rust and provide protective film in the form of an oil surface.
Methods for removing rust depend on the degree of damage:
- cleansing of elements by complete immersion in the product;
- surface treatment using a roller or spray;
- applied to hardware after mechanical cleaning.
Purification of elements by complete immersion in phosphoric acid
If you have a sufficient amount of the product, you can use the full immersion method to remove rust. To do this, you first need to degrease the element to be cleaned using a detergent composition, and then wash it thoroughly. After this, pour 100 grams of an 85% acid solution into the prepared container and add 1 liter of water. The element to be cleaned is immersed in the solution for one hour, stirring the product periodically. Then the product must be removed and washed well.After such cleaning, the product is washed with another solution consisting of 50% water, 2% ammonia and 48% alcohol. Then the product is washed again with plain water and dried.All stages must be performed in strict sequence. If you do not degrease the product, the cleaning will be uneven, since the product may not corrode normal stains, and additional cleaning will be required.In this way, you can clean any products with varying degrees of rust, but the thicker the coating, the more time you need to keep the element in the solution.If the element is not dried after washing, hydroxide appears on it. Drying can be done using any method.
Cleaning rust by applying phosphoric acid to the surface
If the product cannot be immersed due to its size or there is a small amount of product available, then the method of applying acid to the surface with a spray, roller or brush is used. But first it is necessary to indicate the extent of the damage.
If there is significant plaque, you must first remove upper layer using a grinder and a metal brush. Then the surface is degreased and a solution of orthophosphoric acid is sprayed. In this case, the solution should be applied to the entire affected surface, without gaps. After two hours, wash the area with a neutral solution. At the end, a final rinse is performed and the treated area is thoroughly dried.
If the rust layer is small, you can skip cleaning with a brush, but immediately begin degreasing and applying the solution. But re-spraying may be required if cleaning is not complete.
Using orthophosphoric acid, you can clean bathtubs, washbasins and toilets; it completely cleans rusty deposits on enamel surfaces. For products made of acrylic material, such cleaning is not acceptable.
For enameled and earthenware products, you need to dilute 100 grams of acid with 500 milliliters of water. First, the surface is degreased using a detergent and thoroughly cleaned from household contaminants. Then apply acid and wash it off with soda solution. To do this, dilute 1 spoon of soda in a liter of water. In this case, no friction is produced, as a result the enamel surface retains its integrity.
When using acid, do not allow it to come into contact with the skin, otherwise it is necessary to rinse the area with water for a long time.

How to properly store and transport phosphoric acid
Phosphoric acid is an aggressive agent, so it is necessary to follow certain rules when transporting and storing this substance. The powder must be placed in an airtight container. To prevent foreign elements from getting into the acid, all containers must be used clean and dry, then the prepared solution will be of high quality.
Preparing a low-quality composition can result in dangerous vapor poisoning or lack of the desired result. Store containers with acid in a dry and warm place where there is no dampness or condensation. There is no need to pour the powder into another container; it is better to leave it in the original packaging. Such cargo is considered dangerous, therefore, to transport it by long distances, you will need special documentation.
Cleaning is done with care so that the metal surface does not become too thin and holes are formed. During preliminary mechanical cleaning, disks with large elements cannot be used, otherwise significant damage to the surface can be caused.
Before starting the main work, the remaining surface must be covered with film, since as a result of exposure to such strong remedy The rest of the coating may be damaged. Therefore, processing must be carried out carefully so that the damaged surface does not have to be restored, which will lead to additional costs.
If the work is done correctly, the result is a reliable and durable surface that does not contain rust stains, which lead to the destruction of metal products.
When applying orthophosphoric acid to a surface, it is necessary to wear gloves and a respirator; they serve as means of protection against harmful substance. If acid gets on clothing, it must be removed immediately so that the product does not get on the skin and cause a burn.
Basic rules when using phosphoric acid to remove rust
- Before starting the main work, it is necessary to prepare all protective equipment, since the substance is aggressive and leaves burns if it comes into contact with the skin.
- First, the surface must be cleaned of normal contaminants so that the product can perform its functions fully, otherwise the surface will be cleaned in parts.
- If the rust layer is too thick, then it is necessary to resort to mechanical cleaning surfaces, for this they use a grinder and a metal brush.
- The cleansing area must be degreased and then rinsed with water so that the product adheres well and works effectively.
- After finishing the cleansing, the acid must be washed off with a neutral solution and then with water.
- Since the substance is aggressive, it can damage the main surface, so it must be covered with film.
- If it is not possible to clean by immersion, then use sprayers or apply acid with a brush or roller to the rusty surface.
- If the surface is not clean after the first application, additional spraying will be required. After this, the area to be cleaned must be washed with a neutral solution and then with water.
- Phosphoric acid cannot be used to clean rust on acrylic surfaces.
Benefits of Phosphoric Acid for Rust Removal
- Phosphoric acid removes rusty deposits well on enamel and earthenware products, metal surface, is actively used in the automotive industry.
- Using this product you can remove rust without rubbing the surface, this helps preserve the enamel.
- Phosphoric acid forms a film on the cleaned area, which serves to protect the surface from new corrosion and other damage.
- This acid can be used to clean bathtubs, toilets and washbasins, as well as other household appliances that have a similar surface.
- Phosphoric acid helps remove rust stains and subsequently protect the surface from corrosion damage.
When working with acid, it is necessary to wear protective equipment, a respirator and gloves, as the substance is aggressive. Before applying acid, the surface must be degreased using detergents, then rinsed well with water, and then sprayed with acid. After the required time has passed, the acid is washed off with a neutral solution based on alcohol, ammonia and water. After this, the surface is washed with water and dried. If the rust layer is too thick, then it must be cleaned first. wire brush or a grinder with a special attachment. The work must be done in correct sequence, if you skip at least one step, this will lead to poor-quality cleansing.
One morning you leave the house and discover a red spot on your “almost” brand new car, which appeared at the site of a recent chip or crawled out from under the swollen paint. Or maybe you purchased a used car that already has traces of rusty metal. Rust is the corrosion of iron and its alloys that occurs when they come into contact with oxygen in a humid environment. The substances formed during this reaction are iron oxides. Their peculiarity is that, appearing at the top of the metal, they do not create a barrier for the underlying layers of material (and they are also susceptible to corrosion). Over time, rust spreads around the original stain and deeper into it. If you do not prevent this, then after a certain period of time your car will simply fall apart.

Simply painting over the rust will not work. Or rather, it will work out, only the iron oxide remaining under the paint, like a virus, will infect another, “healthy” metal. Therefore, the first stage of restoring a car’s paintwork is to remove existing rust. There are several ways.
In this article we will pay attention to the chemical, which is based on the use of phosphoric acid. Its use against rust is widespread.
Where to buy phosphoric acid?
In auto chemical goods, electronics, radio equipment stores, you can find it at construction markets, and, of course, order it on the Internet. Phosphoric acid is sold where other acids, paints, solders, and various reagents are also sold. It is rarely found in its pure form; more often it can be found as part of a rust converter based on orthophosphoric acid (almost all).
What is phosphoric acid?
This is an inorganic compound that is highly soluble in water. Used in Food Industry(as part of chemical phosphate fertilizers), in medicine (dentistry), in production household chemicals(as the main component for rust converters and metal primers).
Removing rust with phosphoric acid

Removing rust with phosphoric acid
Possible in three ways:
- Complete immersion of the element being processed in a solution of orthophosphoric acid;
- A combination of chemical and mechanical rust removal methods (mechanical cleaning plus treatment with phosphoric acid);
- Treating the problematic surface with orthophosphoric acid using a spray gun or roller (one or more times).
How to dilute phosphoric acid to remove rust?
Phosphoric acid, unless it is part of a rust converter, is sold in powder form. In this case, the question arises: “How to dilute phosphoric acid with water?” The ratio of water to acid should be approximately 1/5, 1/6 (85% solution). Having prepared such a solution, we can say that we made a rust converter from orthophosphoric acid with our own hands. It will not be possible to make a more complex converter at home, since manufacturers carefully hide the composition of their products.
If you are afraid of making a mistake in your calculations, you can purchase ready-made, that is, diluted orthophosphoric acid in syringes, but its price is actually more expensive.
The use of phosphoric acid against rust in a car

Treatment of metal with orthophosphoric acid before painting
Treating the metal with orthophosphoric acid before painting is a must when working to restore the paintwork on a car (can be in the form of a primer or converter). Phosphoric acid removes loose corroded iron by corroding it. Once exposed to rust, it forms a thin protective layer in the form of an oil stain.
If acid gets on moisture-permeable clothing, it is recommended to remove it in order to eliminate the possibility of acid getting on the skin.
This is very useful property, but at the same time very dangerous for a person in contact with this substance. Before work, you should protect your hands with gloves and your respiratory system with a respirator.
It is not advisable to treat thin surfaces with phosphoric acid, as holes may form. The areas adjacent to the problem spot should be protected with a special film so as not to injure the entire paint.
If you work carefully and follow all the necessary rules for removing rust with phosphoric acid, you will achieve the desired result (complete removal of rust and protection of the metal from further destruction).
Analysis chemical composition and the impact of its components as a whole on the human body using the example of the highly carbonated drink “Coca-Cola”.
Coca-Cola is one of the most recognizable and famous drinks of our time. But who knows what chemistry is hidden in the composition of this drink and what else it can serve in life, besides quenching thirst.
Plan:
1.Composition and calorie content of the product.
2. The influence of the components of the product and the product itself on the human body.
3.Use of the drink in everyday life.
4. Conclusions from the above.
1.1 Composition.
The composition of Coca-Cola is as follows:
Sugar, or sucrose (C12H22O11)
Sugar color dye (E150)
Phosphoric acid (H3PO4)
Caffeine(С8H10N4O2)
Some flavors
Carbon dioxide(CO2)
1.2 The nutritional value drink
Calorie content 42 kcal
Proteins 0
Fats 0
Carbohydrates 10.6 g
Sodium<11,0 мг
Potassium 1.0 mg
Calcium 4.0 mg
Magnesium 1.0 mg
Phosphorus about 17 mg
2.1 The effect of orthophosphoric acid (E-338) on the human body.
Phosphoric acid (E-338) is used as an acidifier in various drinks (in fact, not only in Coca-Cola). Causes stomach upset.
Phosphoric acid has a pH of 2.8 (a 5% sulfuric acid solution has a pH of 2.5). Derivatives of orthophosphoric acid are also used in the food industry - from baking powders and the preparation of processed cheeses to sausage production and sugar production. The main area of use of orthophosphoric acid is the production of phosphorus and complex concentrated fertilizers, the production of feed phosphates, synthetic detergents and water softeners.
Phosphoric acid is often labeled on packages as “acidity regulator E-338.”
Phosphoric acid disrupts the acid-base balance in the body towards increased acidity. To neutralize it, the body has to displace calcium from bones and teeth. Hence the caries. The same reason leads to an earlier and earlier onset of osteoporosis.
Orthophosphoric acid at high concentrations causes burns, vapors cause atrophic processes in the nasal mucosa, nosebleeds, crumbling of teeth, changes in blood flora, etc. When consumed, it causes digestive tract upset and vomiting.
2.2 The effect of caffeine on the human body.
Even though the amount of caffeine in Coca-Cola is not that high, it is still necessary to be aware of the harmful aspects of repeated consumption of this ingredient.
High levels of caffeine in the body overload the adrenal glands, resulting in a chronic state of mental stress.
Caffeine also increases blood pressure, which is usually accompanied by severe headaches. This situation is made worse by the fact that stopping caffeine intake (for example, in the form of coffee) can also cause headaches.
When consuming more than 250 mg of caffeine, there is a risk of various ailments, a list of which is given below. (If, in addition to memory deterioration, you find symptoms of five or more of the listed diseases, then memory problems are likely due to insufficient attention caused by consuming large quantities of caffeine.)
Diuresis (feeling of needing to urinate frequently).
Excitability.
Blood flow to the face.
Gastrointestinal disorders.
Insomnia.
Convulsive muscle twitching.
Nervousness.
Incoherent speech.
Cardiopalmus.
Dysphoria (negative emotional state of irritability or anger).
This situation is aggravated by the fact that as soon as the effect of caffeine ends, a loss of energy sets in, which often leads to headaches, fatigue and certain problems with concentrating and remembering new information.
2.3 The influence of the gas contained in the drink on the human body.
Carbon dioxide (CO2) is a heavy, colorless and odorless gas compared to air. The impact of its increased concentrations on living organisms classifies it as an asphyxiating gas. Slight increases in concentration up to 2-4% in unventilated areas lead to the development of drowsiness and weakness. Dangerous concentrations are considered levels of 7-10%, at which suffocation develops, manifesting itself in headache, dizziness, hearing loss and loss of consciousness for a period of time from several minutes to one hour. Poisoning with this gas does not lead to long-term consequences and after its completion the body is completely restored.
2.4 The effect of the drink as a whole on the human body.
Any specific negative effect on the body of the drink has not been officially established. The effect of the Coca-Cola drink on health is no different from other similar products. Thus, it is not recommended to drink highly carbonated drinks for persons suffering from diseases of the gastrointestinal tract, in particular, acute and chronic gastritis, including those accompanied by increased gastric secretion, peptic ulcer of the stomach and duodenum, disorders of the biliary tract, diseases of the pancreas and other pathological processes. Diabetes patients should be aware of the sugar content in classic types of drink.
There is mention of a study that found a link between regular daily consumption of classic Coca-Cola and an increased risk of developing type 2 diabetes.
The head of the epidemiology department of the National Institute of Environmental Health Research (USA), Dale Sandler, studies the impact of food on human health. Dr. Sandler's research found that obesity, a history of kidney stones, and drinking colas may increase the risk of developing kidney disease.
3.1 Using Cola you can do the following:
Remove rust. Coca-Cola is an excellent rust remover. If you have household items that are covered in rust, soaking them overnight in Coca-Cola and scrubbing them thoroughly the next morning will amaze you with their appearance. The properties of cola help to destroy rusty particles, which makes cleaning much easier. You can also use this drink to clean fabric from rust; just pour a little cola onto the stain and rub in in a circular motion.
Wash the windows. Citric acid (C6H8O7), in addition to removing rust, is very effective in cleaning windows. It is especially useful when cleaning car windows. After the procedure, do not forget to wipe the glass with a damp cloth to remove any remaining sugar. Coca-Cola in this case acts as a cheap alternative to many citrus cleaning products, the price of which is much higher than a can of the famous drink.
Eat her. Cola is often used as an ingredient in the preparation of various dishes. You can mix it half and half with your favorite sauce and marinate the chicken in the resulting mixture. The sugar in the cola will give the chicken a glossy coating and caramel flavor, and the citric acid will give it a nice flavor.
Get rid of unpleasant odors. If an unpleasant odor of unknown origin has settled in your house or apartment, then adding a little cola to a bucket of detergent and washing the floor with it, you can easily get rid of the unpleasant odor. Moreover, if you yourself are saturated with an unpleasant odor, then by dousing yourself with cola and then washing it off with plain water, you can easily get rid of it. An added bonus is the fact that cola has a beneficial effect on hair.
Numb the bite. The chemicals contained in cola can be very effective in neutralizing the pain of a jellyfish sting. Most likely, most people are unlikely to carry special pain-relieving lotions with them to the beach, but you can always find a bottle of cola. A small amount of it should be poured onto the bite site, and you will immediately feel relief.
Clean the dishes. Sometimes the bottom of pots and other utensils becomes covered with a black film that is almost impossible to remove. It appears due to burning food. To remove such black marks and restore the appearance of the dishes, pour a can of cola into it and place it on the stove with low heat. After about an hour, remove from heat and wash as usual.
Wash clothes. Some stains are very difficult to remove from clothing, and stain removers are expensive. But there is a cheap solution to the problem: mix a can of Coca-Cola with regular powder and run a regular wash cycle. Using this method, you can effectively remove even blood stains, as well as deodorize unpleasant-smelling clothes.
Get rid of bugs in the garden. Pour the cola into a shallow container and place it in the garden or vegetable garden near the problem area. Slugs, snails and other bugs, if they crawl in there once, they will never get back out. This will significantly save you wasting money on pesticides. You can water cola plants that like acidic soil, such as azaleas and gardenias.
"Make" an explosion. Most people familiar with the Internet have probably heard about the interaction of cola with Mentos. The idea is that if you put one Mentos mint into a bottle of Coca-Cola, the resulting chemical reaction will result in a relatively powerful explosion.
4. The conclusion can be formulated as follows: Coca-Cola is one of the most harmful highly carbonated drinks that are now sold on store shelves. Its manufacturers have produced an excellent pain reliever and an equally bad dishwashing detergent, but not a thirst quencher (it is a known fact that this drink, in turn, even stimulates it). So, people, drink proven products, not chemicals created for the sake of making money!
Phosphoric or orthophosphoric acid was discovered by R. Boyle by dissolving the white substance resulting from the combustion of phosphorus in water. Orthophosphoric acid (chemical formula H3PO4) is an inorganic acid and, under normal conditions, in its pure form, is represented by colorless rhombic crystals. These crystals are quite hygroscopic, do not have a specific color, and easily dissolve in water and in many different solvents.
 Main areas:
Main areas:
- organic synthesis;
- production of food and reactive acids;
- production of phosphorus salts of calcium, sodium, ammonium, aluminum, manganese;
- medicine;
- fertilizer production
- metalworking industry;
- film production;
- production of activated carbon;
- production ;
- production of detergents;
- match production.
Phosphoric acid is of great importance for plant nutrition. They need phosphorus to produce fruits and seeds. increase the productivity of agricultural crops. Plants become frost-resistant and resistant to adverse conditions. By influencing the soil, fertilizers contribute to its structuring, suppress the formation of harmful organic substances, and favor the development of beneficial soil bacteria.
Animals also need phosphoric acid derivatives. In combination with various organic substances, it takes part in the metabolic process. In most animals, bones, shells, needles, teeth, spines, and claws consist of phosphorus derivatives found in the blood, brain, connective and muscle tissues of the human body.
 Orthophosphoric acid has also found application in industry. Wood, after impregnation with acid and its compounds, becomes non-flammable. Thanks to this, the production of fire-retardant paints, non-flammable phosphate foam, non-flammable phospho-wood boards and other building materials has been established on its basis.
Orthophosphoric acid has also found application in industry. Wood, after impregnation with acid and its compounds, becomes non-flammable. Thanks to this, the production of fire-retardant paints, non-flammable phosphate foam, non-flammable phospho-wood boards and other building materials has been established on its basis.
If phosphoric acid comes into contact with the skin, it causes burns; in acute poisoning, it causes vomiting, headache, dizziness, and difficulty breathing. Its vapors, when inhaled, irritate the mucous membranes of the upper respiratory tract and cause coughing.
Orthophosphoric acid is a food additive assigned code E338, which is included in drinks based on flavorings. It is also used in the production of meat and sausage products, processed cheeses, sugar production and bakery.
 Abuse of which contains orthophosphoric acid is completely unhealthy. The harm it causes to humans is to increase the acidity of the body and disrupt the acid-base balance. “Acidification” of the body is a very favorable environment for various bacteria and the process of decay. The body begins to neutralize acid with calcium, which is borrowed from bones and teeth. All this leads to the development of dental caries and bone fragility. The risk of bone fractures increases and early osteoporosis develops. Due to excessive consumption of E338 in food, the normal functioning of the gastrointestinal tract is disrupted. The daily dose for human consumption has not been determined.
Abuse of which contains orthophosphoric acid is completely unhealthy. The harm it causes to humans is to increase the acidity of the body and disrupt the acid-base balance. “Acidification” of the body is a very favorable environment for various bacteria and the process of decay. The body begins to neutralize acid with calcium, which is borrowed from bones and teeth. All this leads to the development of dental caries and bone fragility. The risk of bone fractures increases and early osteoporosis develops. Due to excessive consumption of E338 in food, the normal functioning of the gastrointestinal tract is disrupted. The daily dose for human consumption has not been determined.
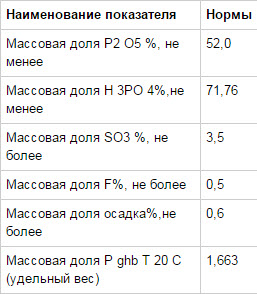
Chemical formula: P2O5nH2O.
International terminology: phosphoric acid
Packaging and delivery: in cans.
Price: piecework-negotiable.
Concentration: (75.85%)
Orthophosphoric acid is a yellow solution in appearance; at 20 degrees Celsius it behaves like a solid material (colorless hygroscopic crystals), when temperatures above 213° Celsius it turns into pyrophosphoric acid H4P2O7. Orthophosphoric acid is supposed to be considered an 85 percent water-based solution (colorless and odorless liquid).
It dissolves well in ethanol, water, and many other solvents.
Application:
In the food industry it is used for the production of baking powders, processed cheeses, sausages and sugar. Phosphoric acid is also found in drink flavorings, such as Sprite, Coca-Cola, Pepsi-Cola and many others. Contained as an antioxidant (E338) in sparkling water, cookies and crackers.
Its main area of application is the production of phosphorus and complexly concentrated fertilizers, feed phosphates, artificial washing and water softening agents, phosphorus salts of ammonium, calcium, sodium, aluminum and manganese.
For the synthesis of activated carbon and the production of film, refractories, glass, ceramics, in medicine, in metallurgy for the purification of metals, in the textile industry for the production of fire-retardant fabrics, in the oil industry, for the production of food and reactive phosphoric acid.
Used in mechanical engineering and the automotive industry as a corrosion converter. Creates a protective film on the treated surface, preventing rust. It is also used in freons and refrigeration devices.
It has a beneficial effect on the soil, promoting the emergence of bacteria and suppressing harmful organic matter, which has a positive effect on the quality of the crop. Used in fur farming.
This is an invaluable product for the chemical industry. Currently, not a single production specializing in fertilizers can do without using it as a basis for the production of phosphorus-complex fertilizers and technical phosphates.
It is transported in railway tanks and stored in closed, sealed containers and plastic canisters. When using phosphoric acid, you must follow the rules of caution, since the substance is dangerous when it comes into contact with the skin; it forms burns and causes skin diseases).
(H3PO4) is a non-flammable liquid substance, therefore fire and explosion-proof.