There are three ways to remove rust from the surface of ferrous metals: thermal, mechanical and chemical. For the most part such metal cleaning is combined - thermal with mechanical, mechanical with chemical, etc.
thermal method
It consists in heating the product gas burner or in the mountain. Rust lags behind the metal surface and is cleaned with a wire brush.
mechanical way
It is used to remove oxides from simple-shaped parts, all places of which are accessible for processing with a steel scraper, wire brush and coarse skins.
Good results are obtained by the use of fish oil, which is rubbed on a rusty surface. The rust layer impregnated with fat is easily removed. Accelerates the cleaning of the use of round brushes (brush brush), driven by an electric drill or electric motor.
A brushing brush is made of steel wire with a diameter of 0.2-0.4 mm, which is tied in bundles around the base ring with a diameter of 1/4 of the future diameter of the brush.
1- base ring; 2 - wire bundles; 3-
soft wire belt; 4 - ring gaskets;
5 - disk; 6 - bolt.
Each bundle of wire from the outside close to the base ring is taken with a belt of soft wire. Thus, a round wire brush is formed, which is placed between two discs with a diameter of about 1/2 of the diameter of the brush. By itself, the edges between the disks and the bundles of the brush on both sides install ring gaskets, otherwise the thickening at the place where the bundles of wire wrap around the base ring will not make it possible to pinch the wire of the bundles. The free ends that protrude beyond the discs should represent approximately 1/4 of the brush diameter. If the ends of the wire are short, the brush cleans the surface better. The assembled brush is constrained in a vice and charged with three bolts or riveted, and a hole is drilled in the middle for fastening. You can tighten everything together with a nut on the shaft, and then clamp it in the chuck of an electric drill.
Before connecting the parts of the brush, it is necessary to degrease and fill the space between the wire bundles and the disks with epoxy resin (in this case, the disks can not be riveted). After the resin hardens, each wire is fixed, less bending.
The rotation of the brush with a diameter of 130-150 and 250-270 mm is allowed at a frequency of 2800 and 2100 rpm, respectively.
Often, a brushing brush is mounted on the shaft of a three-phase motor. When such a motor is connected to a single-phase electrical network, one of its outputs is connected to the direction network, and the other two are connected to the outputs of a K73G capacitor designed for a voltage of 400-600 V, and then another wire is connected to the network from any output of the capacitor. If the motor turns in the wrong direction, then the wire is connected to another terminal of the capacitor. A 1 kW three-phase motor requires a 20 uF capacitor or a set of smaller capacitors connected in parallel. When running a three-phase motor alternating current from a single-phase power supply, it loses about a third of its power.
Chemical method
It lies in the fact that the rusty surface is etched in weak solutions of acids or their reagents. If the product is dirty, it is first washed in hot water, and coated with lubricating oils or fats, it is degreased. Then the part is poisoned, neutralized and passivated.
Parts contaminated with fats are degreased with alkali solutions of 5-10% concentration or organic solvents. Use this solution, parts by weight:
Caustic soda…………………………………………………………………….2.5-3.0
Trisodium phosphate…………………………………………………………..1.5-2.0
Emulsifier OP-7, OP-10 (or washing powder)………0.2-0.3
Defoamer PMS-10……………………………………………1
Soda ash……………………………………………………..100
You can use other alkaline solutions with an admixture of potash or soda up to 3%. The indicated components are dissolved in hot water, the products are immersed in a degreasing solution for 5-10 m. After that, the alkali residues are washed off with a jet hot water for 1-3 m.
The industry also produces ML-51, ML-52, MS-5, MS-8, ML-2 pastes and TMS-31 detergents, from which a 3-8% solution is prepared, which is heated to a temperature of 65-70 ° C . Mineral oils and some conservation lubricating oils are washed off in ML-51 and ML-52 solutions. Aluminum is treated with ML-2 solution, and mineral oils and polishing pastes are removed with TMS-31.
For density, add to rare alkaline solutions slaked lime or magnesia until the consistency of a rare porridge is formed. It is applied with a brush on the product and washed off after 3-6 minutes.
Details and products with gaps and channels, cadmium and zinc, as well as contaminated with mineral oils and tar, are washed off several times with organic solvents (immersed or washed with brushes), each time taking a fresh solvent. If the part is immersed or washed only once, the washed-out oil dissolves and covers it with a thin film.
Oxides are etched in acid solutions with an admixture of inhibitors, pickling additives or pickling regulators, due to which pure metal is almost not destroyed, and only rust is corroded.
The industry produces ChM, PB-5 inhibitors, etc.
As simple inhibitors, products of organic origin can be used - brewer's yeast, sour beer, rye flour, sugar production waste, etc. Inhibitors are also urotropin (dry alcohol), chromates, nitrites and urea. It should be noted that inhibitors act not only in solutions of acids and alkalis, but also in a neutral environment, since they form a protective film on the surface of pure metal. In addition, when pickling a metal without an inhibitor, the hydrogen that is released in this case saturates the surface, as a result of which it becomes brittle. An ammonia-formalin inhibitor is prepared from a mixture of 40% formalin (8 parts by weight) and 25% ammonia (5 parts by weight).
An inhibitor from a solution of iodine in potassium iodide is prepared as follows, parts by weight:
Potassium iodide…………… 1
Crystalline iodine ....0.5
Water distilled…… 10
Acid pickling agents. A solution with an inhibitor for the digestion of ferrous metals can be prepared from such substances, parts
for mass:
Sulfuric or hydrochloric (concentrated) acid .... 5-10
Water …………………………………………………………………… 90-95
ChM inhibitor …………………………………………………..0.1-0.25
Instead of an FM inhibitor, you can use ammonia-formalin or PB inhibitors - respectively 2.5 or 0.1-0.25 parts per mass, as well as urotropin (dry alcohol), a solution of iodine in potassium iodide - respectively 0.5, 0.75 and 1-1.5 parts by weight.
When preparing a solution of sulfuric acid, be sure to pour acid into water.
The FM inhibitor is used mainly with sulfuric acid, and PB - with hydrochloric acid. If there are no concentrated acids, weaker ones are used. For this, the amount of water is reduced accordingly, so that a 5-10% solution is formed. top scores than sulfuric or hydrochloric acids, gives etching with phosphoric or orthophosphoric acids. After them, a phosphate film is formed on the metal surface, which prevents corrosion for a while.
It is best to pickle small items in glassware, and large ones - in wooden containers, covered inside with asphalt, settled upper part of mastic for protective coating of car bottoms or plastic lining. Plastic 50-liter cans are also convenient for pickling, from which the upper narrow part is cut off with a saw. The part is lowered into the solution and kept in it until all rust is removed. Products that cannot be immersed in a solution, such as sheets of black plaque, are treated with a paste that is prepared from two solutions. The first includes, parts by mass:
Hydrochloric acid ……………………………..16-17
Inhibitor (urotropin)……………………….1-6
Filler (shredded paper)…….4
Water………………………………………………………50
When preparing the first solution for the paste, instead of technical hydrochloric acid, you can take the same amount of inhibited hydrochloric acid and do not add an inhibitor to it. The second solution is prepared from liquid glass and water (respectively 5 and 15 parts per mass).
The second solution is poured into the first solution, mixed well and settled for a day. During this time, the mixture will thicken to the consistency of a liquid gel. It is applied to a degreased surface with a layer of 1.5 mm with a paint brush or spatula and kept for 15 minutes to 12 hours. The paste is removed together with rust, a spatula, and the surface is wiped with acetone or another solvent. For 1 m2 of metal surface, 1-1.5 kg of paste is used. The etching rate depends on the solution temperature, concentration and type of acids. So, in a 10% hydrochloric acid solution at a temperature of 18 ° C, etching lasts 18 minutes, at 40 ° C - 6 minutes, at 60 ° C - 2 minutes, and in a sulfuric acid solution - 120, 32 and 8 minutes, respectively. Although etching in sulfuric acid lasts longer than in hydrochloric acid, nevertheless, its cost is 5-6 times less and it costs much less. Therefore, when processing a large number of products, it is more expedient to use sulfuric acid. Solution for etching parts with exact dimensions such, parts by weight:
Chromic anhydride…………..4-5
Orthophosphoric acid …..1
Water………………………………..20
At a solution temperature of 90-95°C, etching lasts 1-2 hours. Products can be etched with a Rust Converter solution. A solution for removing rust from valuable steel products is prepared from the following substances, parts by mass:
Tartaric acid……………10
Water ………………………………………..40
Inhibitor (possible without it)…..0.1-0.15
Tartaric acid………………1
Zinc chloride………………………..10
Water …………………………………………100
It is easy to prepare such an etching solution, parts by weight:
Orthophosphoric acid……………….2
Acetone……………………………………..2-12
Hydroquinone……………………………0.08-0.1
Water……………………………………………10
Products are kept in solutions until the rust disappears, and then washed in water (temperature 60-60 ° C), in which 1.5-5.5 g is dissolved soda ash and 0.5-5 g chrompic. Passivation of products made of ferrous metals is necessarily used after etching them in acid solutions, otherwise they will rust again. During this treatment, the surface of the parts is formed protective film which partially prevents rusting. If metal surfaces are covered after rust removal protective coating, they are not passivated.
For passivation, a solution is prepared from chromic and caustic soda, parts by mass:
Chrompeak ………………….8
Caustic soda ……1
Water ………………………..1
Well-washed after pickling products are immersed in this solution for 15-15 minutes, removed and washed again, and then dried. It can be passivated only in a 10% chrompic solution, i.e. without caustic soda, but with room temperature(withstand 1 hour).
More effective will be a solution of sodium nitrite in water - respectively 8 and 10 parts per mass (treated for 20 minutes at a temperature of 30-30 ° C).
Acid-free rust removers. zinc chloride solution. An arbitrary amount of zinc chloride is taken (depending on the size of the product) and distilled water is gradually added to it with stirring until all the powder is dissolved, i.e. prepare a saturated solution. Fat-free parts are placed in the solution for 10-12 hours. After removing the rust, they are washed well with water and wiped with a dry cloth. Smooth surfaces while becoming shiny.
Two solutions are prepared simultaneously from blood salt, parts by weight:
Solution ……………………….1
Yellow Blood Salt….1
Water …………………………….5
Solution ……………………….2
Yellow Blood Salt….1
Cute economic …….1
Chalk ……………………………..2
Water…………………………….20
Products are moistened first with the first solution, and then with the second. After 7-8 hours, when the rust dissolves, they are washed with water and wiped.
I am preparing the UAZ for painting, for this it is necessary to remove the rust, and prepare the surface for primer and Galvanol,
Galvanol
which I want to try on the cockpit.
Help Tsynar,



orthophosphoric acid
Runway Rust Converter,

simple Nizhny Novgorod cleaner for 20 rubles. for 0.5 l.,

A simple Nizhny Novgorod cleaner for 20 rubles.
and some other concentrate 1 liter per 3 liters of water

Some kind of concentrate for dilution with water.
.The rust itself does not come off, even after removing the loose layer with a grinder with a brush.
Since I had nothing to do with rust before, I decided to start with the most famous and inexpensive remedy - Tsinkar.
Tsinkar - I didn’t get a special effect, they hissed a little, the foci bubbled up, I took off just a little bit, three passes with a grinder gave a small result, but the deep pores remained in rust. - 500 ml is over.
There are disadvantages: it does not wash off very well.
The next day, disappointed, I went to the store and bought a B-52, ortho phosphoric acid soldering iron, and a liter of standard cleaner.
I smeared the remains in one place where there were B-52 pores and in another with a rust cleaner, I decided to leave it for a day.
Results:
B-52 - I ate rusty acne up to 0.5 mm, from the second or third time the pores shone, there is no rust, I'm satisfied. But every 30 minutes, I had to rub the grinder with a brush. Very laborious, but the result is.
But there is a drawback that the first layers of dry rust are reluctant to remove.
Therefore, before using it, you need a liquid rust destroyer, and b-52 for stripping to metal.
In another place, a liquid cleaner - from the fifth time, constantly pouring it into the sore spot, the rust disappeared by almost 90% of the deep pores processed B-52 and cleanliness.
When the 1st can of B-52 ran out, I went to buy more, where I bought it was gone, went to a car painter's shop, where I took the last can.
Heaven and earth compared to the first bottle bought in the household store, the same jelly, only the cork is yellow,
It does not work at all, possibly a fake and a delay. Like this!
I bought another rubbish RunWay Rust converter - a small jar for a lot of money.

smeared the treated surface with a small amount of rust, as instructed, and dried.
The treated area turned blue, the rust turned black and the film dried up.
Result: after 3 days I tore off a piece of this coating, under it the rust remained untouched, i.e. in my opinion, the effect is 0, only the release of money.

Tore off all this film under it rust! I returned to the dreary cleaning of the B-52. cleansed all pores.
I want to try phosphoric acid, pour it on the floor under the driver, there is a lot of rust and deep.
Experimental results will follow later.
I’ll also try a cheap Nizhny Novgorod cleaner, mock them for rust.
So far, the conclusions are as follows, you need to take a cheap liquid cleaner, first we wet it abundantly, clean it, and finally process it to white metal B-52.
Nizhny Novgorod cheap rust cleaner - it is a cheap cleaner, it drains very liquid immediately, after it the rust dissolves a little, gets wet, is removed just like without it, did not come up with a way to make cleaning easier with just this cleaner.
Orthophosphoric acid (soldering) - works a little better than a cheap cleaner, resembles oil in properties. I poured a piece of loose rust on it, after a day it dried or absorbed, the rust turned black but did not disappear. On next day soaked a cloth with acid, and applied it to the affected area, the results became better, the acid did not evaporate, the rust went away by 70% with a hairy brush, the area shone, 20 minutes after treatment and cleaning it became golden. It is necessary after the acid immediately to cover with something.
I cut off the driver's door card for repairs a little more, I'll be a shaman.

Rust on the door

Cut off the lining, and the frame which is in dust.
Photo processing results

Rust Free

TO category:
Preparing for painting
Etching the surface before painting
In cases where the removal of scale by mechanical methods is either impossible (for example, when cleaning products of small thickness, complex configuration), or it is not economically feasible, apply chemical methods, of which the most common is pickling - the removal of scale and rust when exposed to pickling (acid) solutions.
The etching process has some advantages and disadvantages compared to mechanical cleaning methods.
The advantages include: great performance; simplicity of the equipment used and the process; the possibility of processing steel products of any thickness; economy.
The disadvantages of the method is the need for thorough washing of the surface from the remnants of etching solutions, which requires large amounts of washing tap water n special treatment facilities for neutralization or regeneration of pickling production waste.
Thorough washing of the surface from acid residues is especially important before application. paintwork materials, since the presence of salts on the painted surface leads to moisture osmosis and the development of under-film corrosion. The quality of the wash water should be constantly monitored. The content of foreign impurities in the wash water must not exceed 100 mg/l.
When cleaning the surface from scale and rust, the greatest difficulty is the removal of scale, which is formed as a result of the oxidation of metals during high temperature. In this case, iron is covered with three layers of oxides, of which the outer layer, the most oxygenated, in composition approximately corresponds to iron oxide Fe203 (hematite) and, depending on the oxidation conditions, makes up from 2 to 10% of the thickness of the entire layer. The intermediate layer, which makes up from 20 to 40% of the thickness of the entire layer, is magnetite Fe304; directly adjacent to the metal is a layer of ferrous oxide FeO (wustite) with a lower oxygen content than the first two layers. The thickness of the FeO layer is from 50 to 80% of the total thickness. There is much less information about the composition of rust than about the composition of scale. Most often, rust consists of a layer of iron oxide hydrate Fe(OH)2 adjacent to the metal surface and an outer layer of iron oxide hydrate Fe(OH)3. In addition, some salts may be part of the rust.
The need to remove a dense, thin layer of scale, which can serve as some protection during interoperational storage of hot rolled steel, is confirmed by many studies that have shown that about 50% of all damage to the paintwork during operation is caused by scale peeling.
The scale, which seems solid and smooth, is in reality always covered with a branched network of cracks. The electrochemical characteristics of scale and unoxidized metal are different. The scale is an effective cathode and promotes the acceleration of the dissolution of the neighboring parts of the steel surface. So, according to the data, the presence of scale accelerates corrosion processes in marine conditions by 30-40 times. When using a 100 micron thick paintwork under these conditions, the rusting of a product painted by scale begins after three years; when applying a paintwork material on an etched surface - after eight years, on a sandblasted surface - after eleven years.
IN different time A lot of work has been done on painting surfaces with scale, but in no case has it been possible to improve the protective effect of paint coatings. The same applies to rust painting. It was shown that the state of coatings obtained by applying paints and varnishes to rust depends on the pollution of the atmosphere with ions of SG and SO4. The presence of water-soluble salts in rust increases the electrical conductivity of moisture penetrating through the paintwork and intensifies corrosion processes.
Cleaning of the oxidized surface of metals is carried out with pickling solutions, most often with solutions of sulfuric, hydrochloric and phosphoric acids with various additives.
Descaling in acid solutions is not the result of direct interaction of acid with oxides. It has been shown104 that the dissolution of ferric oxides removed from metal surface, is extremely slow. ferrous oxides (for example, wustite FeO) dissolve much faster.
Oxide films are removed from the surface due to the so-called reductive dissolution process, which takes place in areas of the metal with a disturbed oxide layer. The transition of iron cations into the pickling solution is observed precisely in these areas or in places where the oxide lattice is defective, while the bond between the scale and the surface is broken over time, as a result of which the scale exfoliates.
Descaling is facilitated by hydrogen bubbles, the rapid release of which is observed on the entire etching surface. This effect is especially noticeable in sulfuric acid solutions.
Surface areas with more cracks are cleaned faster than areas with denser scale. There is a local dissolution of the surface, the so-called overheating of the metal. The surface pitting that appears in this case adversely affects the protective properties of the obtained paint and varnish coatings: the increased surface roughness makes it difficult to wash it from salts formed as a result of etching, and causes an increased consumption of paints and varnishes due to an increase in the number of applied layers to cover the protrusions of the overetched surface.
It should be borne in mind that the interaction of the cleaned surface areas with the pickling solution leads to unproductive losses of acid and an increase in the amount of salts formed; this negatively affects the speed and quality of etching. Uniform pickling of the entire surface is achieved by introducing corrosion inhibitors into the pickling solutions - inhibitors that slow down the dissolution of the cleaned sections of the steel surface without affecting the rate of oxide removal.
Regarding the mechanism of action of inhibitors, there are a large number of theories, many of whose authors agree on the adsorption mechanism of blocking the most active parts of the steel surface by inhibitors.
According to the data, organic compounds that have an inhibitory effect in acidic media reduce the rate of the hydrogen evolution reaction, resulting in a decrease in the rate of dissolution of metals in acids.
Effective inhibitors slow down the process of steel dissolution by 98-99%, while the cleaning rate does not decrease or decreases slightly. The choice of inhibitors is made in relation to certain acids.
Etching in sulfuric acid solutions
Etching solutions based on sulfuric acid are the most common; This is due to the following features:
a) low cost;
b) low vapor volatility, which is especially important when using baths with a large open surface;
c) the ability to work at elevated temperatures, which significantly reduces the duration of cleaning;
d) the possibility of transporting concentrated sulfuric acid in unprotected tanks (since concentrated acid passivates ferrous metals);
e) reducing acid consumption by removing most of the scale as a result of exfoliation under the action of hydrogen gas.
During pickling, the concentration of acid in the pickling solution decreases and the content of iron sulfate increases, the solubility of which decreases with increasing acid concentration. The etching rate and the quality of the etched surface depend on the content of ferrous sulfate in the pickling solution.
In the presence of iron sulfate in the pickling solution, the rate of dissolution of the metal surface decreases and the solubility of oxides, especially wustite, increases somewhat. According to the data, the smallest losses of the steel substrate are observed when the content of the pickling solution is 100 g/l of sulfuric acid and 450 g/l of iron sulfate. With an increase in the content of iron sulfate, the quality of the surface on which it settles in the form of sludge deteriorates. Like any water-soluble salt, ferrous sulfate contributes to the osmosis of moisture through the paintwork and the development of under-film corrosion. This significant drawback may limit the use of sulfuric acid in preparing the surface for painting.
Temperature is the main factor determining the rate of etching in solutions of sulfuric acid. It has been shown 108 ± 109 that the use of a 15–20% sulfuric acid solution at 45°C and a 5% solution at 55°C results in the same etching rate. Most often, 15-20% solutions of sulfuric acid are used at temperatures up to 75 ° C.
As inhibitors of sulfuric acid solutions, various additives are used, such as FM inhibitors, catapine (a product of petroleum refining), I1-A, and sodium chloride. The concentration of the first three inhibitors is 3-5 g/l, the last one is 50-75 g/l. The best protective effect is provided by catapine, which reduces the dissolution of steel by 98-99% at elevated temperatures.
Disposal of pickling production waste presents certain difficulties. Waste from small pickling plants, consuming no more than 100-150 tons of acid per year, is economically expedient to neutralize with lime. The disadvantages of this method include the complexity of the separation and processing of the resulting gypsum, requiring large areas. Another way is to extract FeSO4-7H20 from iron sulfate solution; for this, the spent sulfuric acid solution is cooled, iron sulfate precipitates from it, and the remaining solution is neutralized with lime and discarded.
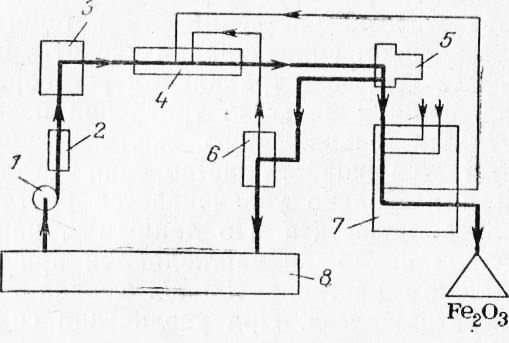
Rice. 1. Scheme of complete regeneration of H2SO4: 1 - pump; 2 - filter for acid; 3 - evaporator; 4 - reactor; 5 - centrifuge; 6 - gas collector; 7 - kiln; B - pickling plant
The unproductive loss of sulfuric acid during neutralization and the difficulties associated with this caused an intensive search for continuous regeneration methods that allow separating iron salts from the main solution, concentrating the acid of spent solutions and returning it to production.
The Austrian company "Rutner" has developed and implemented several schemes for the complete regeneration of sulfuric acid, one of which is shown in Fig. 1.
Etching in hydrochloric acid solutions
About 20% of all pickling plants are reported to operate using hydrochloric acid solutions. Etching with hydrochloric acid solutions achieves a better surface quality than etching with sulfuric acid solutions, which is of great importance for subsequent painting.
The rate of interaction of oxides with hydrochloric acid solutions is much higher than with sulfuric acid solutions, so little sludge is formed in hydrochloric acid pickling solutions.
When etched in hydrochloric acid solutions, a light surface is obtained good quality free of pickling sludge and spotting; the purification rate increases by 2-3 times compared to the purification rate in sulfuric acid solutions; less hydrogen is released, which leads to a decrease in hydrogenation of the metal; steel overtreatment is reduced. The cost of cleaning with hydrochloric acid solutions is much lower than the cost of cleaning with sulfuric acid.
Along with these advantages, the use of hydrochloric acid is also associated with significant disadvantages that reduce its prevalence. These include: high volatility; the need to transport in specially protected tanks (gummed or reinforced with polyethylene), which increases the cost of transportation (this disadvantage is eliminated when transporting inhibited acid); the need to transport large volumes of acid due to the low initial concentration of hydrogen chloride in it.
Despite these shortcomings, the use of hydrochloric acid is continuously increasing due to the use of modern pickling equipment - pickling towers, jet pickling plants, etc.
The spread of the method of etching with hydrochloric acid is closely related to the development special methods complete regeneration of waste solutions, allowing to reduce acid losses up to 2%. One of the schemes developed by iRutner (Austria) is shown in fig. 2.
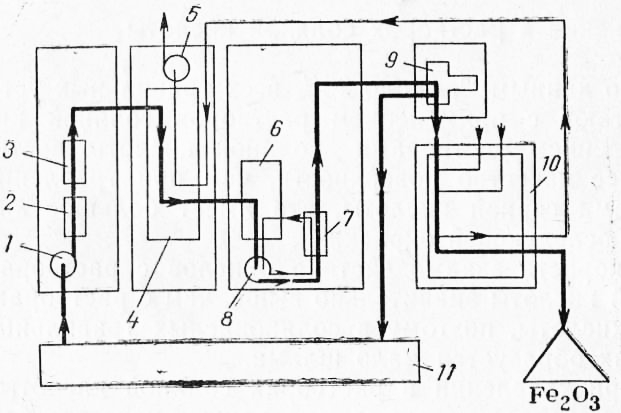
Rice. 2. Scheme of complete regeneration of HC1: 1 - acid pump: 2 - filter; 3 - flow meter; 4 - absorber; 5 -exhaust fan; b - mold; 7 - mold cooler; "- circulation pump; 9 - centrifuge; 10 - kiln; 11 - pickling bath.
The spent pickling solution containing iron chloride dissolved in it is saturated with hydrogen chloride in the absorber. In this case, the solubility of FeCl2 decreases and iron chloride crystals precipitate from the solution when it is cooled in the crystallizer, as a result of which the pickling solution is freed from iron. In the kiln, thermal decomposition of FeCl2 occurs with the formation of hydrogen chloride and iron oxide. Hydrogen chloride saturates the pickling solution, and iron oxide is the end product of the process. The amount of iron oxide corresponds to the amount of scale dissolved in the pickling process.
The spread of the method of etching with hydrochloric acid was facilitated by the use of non-metallic acid-resistant materials (glass fiber, graphite plastic, faolite, etc.), without which the use of modern etching installations would be impossible.
The technological parameters of the pickling process with hydrochloric acid solutions (temperature, concentration) are determined mainly by the materials and design of the pickling plants. Due to the high volatility of hydrogen chloride, it is impossible to work at elevated temperatures, so etching in baths is often carried out without additional heating of the solution. However, equipment sealing makes it possible to use hydrochloric acid solutions of any concentration at temperatures up to 70 °C. This provides a significant reduction in the duration of etching and an increase in the productivity of the installation.
According to the data, the maximum content of iron in the hydrochloric acid solution should not exceed 100 g/l, which corresponds to a content of 200 g/l FeCh.
In hydrochloric acid solutions, overpicking of the steel surface is observed, since with an increase in the content of ferric chloride in the pickling solution, the rate of steel dissolution increases. The introduction of an effective inhibitor significantly reduces overetching and improves the surface quality. In domestic practice, more often than others, hydrochloric acid solutions are inhibited by the composition of PB-5 or catapine (1-3 g/l).
The best quality of the coating and the lower consumption of paints and varnishes are achieved if the etched surface is thoroughly cleaned from metal chlorides.
Sometimes oxide layers from large equipment are removed with hydrochloric acid pastes. Yes, at the Institute organic chemistry The Academy of Sciences of the USSR has developed a cellogel paste suitable for removing rust up to 2-3 mm thick from the surfaces of ferrous metals.
The composition of the paste includes: hydrochloric acid, liquid glass, urotropin, paper pulp or sawdust and water.
Etching in phosphoric acid solutions
Pickling solutions based on phosphoric acid are currently less common than solutions of sulfuric and hydrochloric acids. This is due to the higher cost of phosphoric acid (about 10 times) and the slower rate of descaling. Rust is removed faster with solutions of phosphoric acid than in sulfuric and hydrochloric acids, therefore phosphoric acid is included in almost all cleaners and pastes used to remove rust.
Descaling in phosphoric acid solutions is greatly intensified by stirring the solution or by surface treatment in jet chambers.
Iron phosphate formed during etching has limited solubility in phosphoric acid solutions; the limiting iron content is about 2-2.5%. With an increase in iron content, sparingly soluble phosphates are released, which inhibit further purification. The iron phosphates formed on the steel surface are a good basis for the subsequent painting of the surface without rinsing. Therefore, etching in phosphoric acid solutions is of particular importance when preparing the surface before painting.
At some domestic engineering plants, after etching in solutions of 20-25% phosphoric acid, the surface is not washed. This cannot be considered correct, since after etching in phosphoric acid of such a concentration, a strongly acidic solution remains in the micropores and cracks of the metal surface, which causes the coating to swell, especially in a humid atmosphere.
When pickling in solutions of phosphoric acid, the following sequence of technological operations is recommended: pickling in a 15-20% solution of phosphoric acid at temperatures up to 80 °C; treatment with 2% phosphoric acid solution.
In the first bath, scale or rust is removed, after which the product is transferred without washing into the second bath, where a protective film of iron phosphate is formed within 2 minutes. Initial iron content!
go of the second bath should be 0.3-0.5%, which is achieved by introducing the required amount of steel chips into the solution before processing. Only after treatment with a 2% phosphoric acid solution can the surface be painted without prior washing. The thickness of the resulting film of iron phosphate is ~ 1 μm; therefore, its protective properties are low and it is necessary to color it immediately after drying the etched product.
To remove scale, it is possible to replace phosphoric acid with hydrochloric and sulfuric acids, while the following sequence of technological operations is recommended: etching in inhibited hydrochloric or sulfuric acids; washing; treatment with 2% phosphoric acid solution.
Rinsing of the surface between operations must be thorough, since when transferring acid from the first bath to the third, the surface becomes clogged with water-soluble salts (chloride or sulfate), which, in the absence of a final rinse, adversely affect the properties of the paintwork.
Surface treatment with solutions of phosphoric acid of various concentrations is simple and convenient, but its use is economical only with complete regeneration of phosphoric acid.
The resin treated in this way is able to absorb iron again, and the ferrous sulfate formed is discarded.
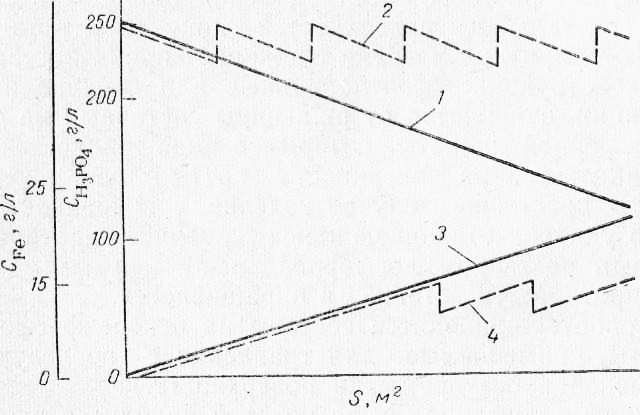
The use of phosphoric acid regeneration is not only of economic importance, but also changes the technological parameters of the process.
During regeneration, the iron content in the pickling solution is not brought to the limit value (about 25 g/l), but is maintained at the level of 10-15 g/l; the concentration of phosphoric acid in the solution is also maintained within a certain range. This leads to a constant pickling rate and a stable surface quality, which is a prerequisite for a good quality of the subsequently applied paint coating.
The regeneration of phosphoric acid with ion-exchange resins makes it possible to widely use it for surface preparation before painting at machine-building plants.
Due to the lower aggressiveness of phosphoric acid compared to sulfuric and hydrochloric acids, its solutions can be used for jet supply without inhibitors.
Large-sized products of a single production, metal structures, assembled or used, are cleaned of rust with phosphoric acid-based compounds, which are applied in the form of liquids or pastes to the surface with a brush or spray. In the simplest case, the product, from the surface of which peeling rust has been removed, is wiped with a 10% solution of phosphoric acid, and then after 24 hours it is wiped with a rag and painted.
The paper1 presents compositions based on phosphoric acid used for etching with subsequent washing and without washing the surface.
The coloring of the surface on which rust is located, using converters, is the subject of many years of research. This is how large-sized metal structures, hydraulic locks, power line supports, fuel storage tanks, etc. are painted. The most widely studied are converters] of corrosion products containing various complexing substances and phosphoric acid.
A wide variety of substances are used as complexing agents; the greatest interest; represent converters of corrosion products based on aromatic hydroxycarboxylic acids (tannins), which are part of natural and synthetic tanning agents.
In addition, tannins have the ability to passivate the metal surface and significantly inhibit the development of corrosion processes. Orthophosphoric acid, which is part of the converters;
rust, dissolves iron corrosion products and increases the efficiency of their interaction with tanks.
The developed converters of corrosion products122 P-1T and P-2 are manufactured by the Riga Paint and Varnish Plant. Corrosion products converter P-1T contains 8-10% phosphoric acid and is recommended for processing corrosion products with a layer thickness of not more than 50 microns. Corrosion product converter P-2 contains a larger amount of orthophosphoric acid and is recommended for processing corrosion products with a layer thickness of 50 to 100 microns; with a larger layer thickness, it is difficult to guarantee the production of coatings with good physical and mechanical properties.
Before processing with transducers, sheet and exfoliating rust must be removed from the surface.
Phosphoric acid rust converters are not effective enough in the treatment of corrosion products containing mainly a-FeOOH (common light-red rust), magnetite (black rust) or a mixture thereof. This is due to the fact that corrosion products of this structure are relatively resistant to mineral acids.
The action of rust converters on surfaces covered with scale is not effective.
The use of rust converters for surface preparation places special demands on top coats; they must have high adhesion to the converted corrosion products, be resistant to acid residues or bind acid residues that are part of the converted corrosion products. There is information about effective application rust converters for painting buried trench fuel storage tanks, building structures, hydraulic structures, etc.
A more universal way of treating corroded surfaces before applying coating materials is priming, which simultaneously provides surface preparation for painting.
The same complexing additives are introduced into the composition of some primers as in the composition of rust converters.
The protective effect of some primers is explained by the stabilization of corrosion products and their transformation into magnetite and hematite. It is generally accepted that when the corrosion products are completely stabilized, an impermeable layer is formed on the metal surface, which is firmly connected to the metal substrate and provides anticorrosive protection of the metal.
The protective properties of coatings obtained by applying paints and varnishes to corroded surfaces, and the processes of phase transformations of corrosion products are determined by the physico-mechanical and physico-chemical properties of paint coatings.
Primer VA-1GP developed at NIITLP is intended for treatment of corroded metal surfaces120. On the metal surface primed with VA-1GP, GF-020, FL-OZk, perchlorovinyl and epoxy primers can be applied. According to the results of accelerated and full-scale tests, the protective properties various systems coatings after surface treatment with a VA-1GP primer are not inferior, and in some cases they are superior to similar systems in which rust converters were used.
Coatings with primer VA-1GP have been successfully tested under production conditions; this primer is recommended for extensive production testing in various industries.
Pickling equipment
The etching process can be carried out in continuous or intermittent baths and in jet chambers. Bath cleaning continuous action applicable mainly for tape. Finished goods or blanks are cleaned in batch pickling baths.
When pickling, the products are immersed in a solution and kept until completely cleaned for 10-30 minutes or more. The pickling process and pickling bath designs are described in the literature121-123. Technological modes of etching in baths are given earlier.
The length of the pickling baths usually does not exceed 12-15 m, the width and height are 2-2.5 m. The bath bodies are made of sheet steel or reinforced concrete and are lined inside with various acid-resistant materials. Reference 124 gives the main recommendations for the design of baths and protection against corrosion of equipment and structures in pickling departments.
Etching in jet chambers is a more advanced processing method than etching in baths. With jet etching, the cleaning speed increases by 2-5 times; the equipment can be sealed and included in production lines for surface preparation and painting. At the same time, working conditions are significantly improved. In addition, when using this method, production areas are reduced, the concentration of pickling solutions is reduced, and, consequently, the consumption of reagents.
In jet chambers, a jet of pickling solution is supplied to the surface to be treated under pressure created by a pump.
Works 125 ± 128 are devoted to the study of the jet etching process. According to NIITLP, the main factor determining the rate of descaling in the jet is the temperature of the pickling solution. Increasing the pressure of the pickling solution jet from 3 to 18 kgf/cm2 leads to the same increase in the cleaning rate, which is achieved by increasing the temperature of the pickling solution from 50 to 60 °C. By changing the temperature of the pickling solution and the length of the jet pickling zone, it is possible to achieve different performance pickling plants.
The concentration of acids during jet etching should be 5-10%. The use of inhibitors is difficult and inefficient. With a decrease in the duration of etching, the overetching of the surface is somewhat reduced. In addition, there is a greater uniformity of cleaning than when pickling in baths. Therefore, solutions during jet etching do not yet inhibit.
In jet pickling installations, acid-resistant structural materials used for the manufacture of pickling baths are of great importance. In the NIITLP unit, the total length of the pickling zone is 6 m. The sections located before and after the pickling sections are enclosed in separate casings so that acid vapors do not affect the parts during other operations.
The bodies of the etching sections are made of fiberglass STEF brand based on epoxy resin ED-6. The manifolds on which the spray nozzles are mounted are also made of fiberglass pipes.
All pipelines of pickling sections are lined with polyethylene; valves are lined with fluoroplastic.
Acid solutions are supplied by a 4x-8f graphite-plastic pump with a mechanical seal. The pump has performed well. Guaranteed service life of seals without replacement - 2000 hours.
Etching solutions are heated in special phaolitic containers by immersing graphite steam heaters. The use of non-metallic acid-resistant materials allows etching with solutions of hydrochloric and other acids at temperatures up to 80 °C.